
Trial Nation offers a single national entry point for life science companies, patient organisations and clinical researchers wishing to sponsor, participate in and conduct clinical trials in Denmark.
Trial Nation offers a single national entry point for life science companies, patient organisations and clinical researchers wishing to sponsor, participate in and conduct clinical trials in Denmark.
Decentralised Clinical Trials
About Decentralised Clinical Trials
Traditionally, clinical trials and testing take place at hospital departments. However, this can be a logistical challenge and time consuming for some participants. It is important that citizens are offered the opportunity to participate in health research regardless of where they live and their level of mobility. Decentralised clinical trials (DCT) are clinical trials designed to take place entirely or partially closer to the participants and their local environment. This can be achieved by using, for example, electronic informed consent, video consultations with doctors, electronic data capture using apps and wearables, home nurses, digital monitoring, local pharmacies, and local GPs and outpatient clinics for e.g. blood sample collection as parts of the trial.
While there are various definitions of DCT, we use the definition:
A decentralized clinical trial (DCT) is a clinical trial that utilizes technology, processes, and/or services that create the opportunity to reduce or eliminate the need for participants to physically visit a traditional research site for some or all of their trial related activities.(DTRA, Decentralized Trial Research Alliance)
Thus, decentralised clinical trials does not have to be all or nothing.
Decentralised Clinical Trials in Denmark
Download an overview of possibilities for conducting Decentralised Clinical Trials in Denmark
For quick and easy information on conducting DCTs in Denmark, download our one-pager. It highlights Denmark’s capabilities in digitalising and decentralising clinical trials and outlines the national strategic focus on DCTs. This document is developed by Trial Nation and partners as part of the PACT project.
Decentralised Clinical Trials and Trial Nation
At Trial Nation, we are dedicated to strengthening patient-centred decentralised clinical trials in Denmark. We accelerate the development of better conditions for conducting DCTs in Denmark by fostering close collaboration between authorities, public and private partners, patients, and researchers.
Trial Nation engage in multiple collaborative activities to stimulate the adoption of DCTs in Denmark:
- Trial Nation is heading a strong national public-private partnership – PACT (Decentralised Patient Centered Clinical Trials).
- Trial Nation facilitates the Danish DCT Dialogue Forum together with the Danish Medicines Agency.
- Trial Nation facilitates biannually meetings with the Danish Research Units for General Practice to accommodate future needs, where more patients are expected to be seen outside of the hospitals (e.g. by general practitioners). Exploring potentials for a stronger collaboration is of high interest to secure the establishment of essential infrastructures to support the conduct of clinical trials closer to the patients.
- Trial Nation collaborate with the Danish Association of Pharmacies. The collaboration aims to develop a concept/framework for the involvement of the Danish local pharmacies in the conduct of clinical trials.
- Trial Nation engage in dissemination and knowledge sharing activities on DCTs
To hear more about Trial Nations activities within DCT please reach out to:
Michelle Rosgaard Birknow
Head of Decentralised Clinical Trials Development
mrb@trialnation.dk
DCT Dialogue Forum
To support the acceleration of DCT in Danish clinical trial settings Trial Nation is facilitating the Danish DCT Dialogue Forum in collaboration with the Danish Medicine Agency. This forum brings together key stakeholders from different sectors – authorities, ethicists, patient organisations, researchers, local and hospital pharmacies, GCP units, and the life sciences industry – to support the use of decentralised elements in Danish clinical trials. The DCT Dialogue Forum meets twice a year to discuss best practices and address challenges related to DCTs in Denmark. It serves as a fast-track platform to raise concerns about DCT frameworks and find solutions. This collaborative effort has been crucial in developing the Danish DCT guideline, which has also influenced the EMA’s recommendation paper on DCTs.
Recommended reading and useful links about Decentralised Clinical Trials in Denmark
To learn more about DCT and the Danish capabilities please visit the following pages.
Visit Danish Medicine Agency here to find the DCT guidance paper about implementation of decentralised elements in clinical trials with medicinal products.
Visit the Danish National Center for Ethics here to find:
Visit the European Medicines Agency here to find the latest versions of:
Visit the European Commission’s website here to find:
Visit Healthcare Denmark’s website here to see interviews with the Danish authorities, clinicians, patients, and the industry sharing insights and perspectives about performing DCTs in Denmark.
Visit Innovation Centre Denmark’s website here to learn about the American DCT ecosystem, and important lessons learned relevant for Danish stakeholders. The full report can be accessed in the end of the page
About PACT-project (Decentralised Patient-Centered Clinical Trials)
The PACT project, led by Trial Nation, is a national public-private collaboration involving key stakeholders in Danish life sciences*. The project is supported by the Innovation Fund Denmark and runs from 2022 to 2026. The primary objective is to make clinical research more available to Danish patients and citizens by promoting better overview, access and conduct of clinical trials in Denmark.
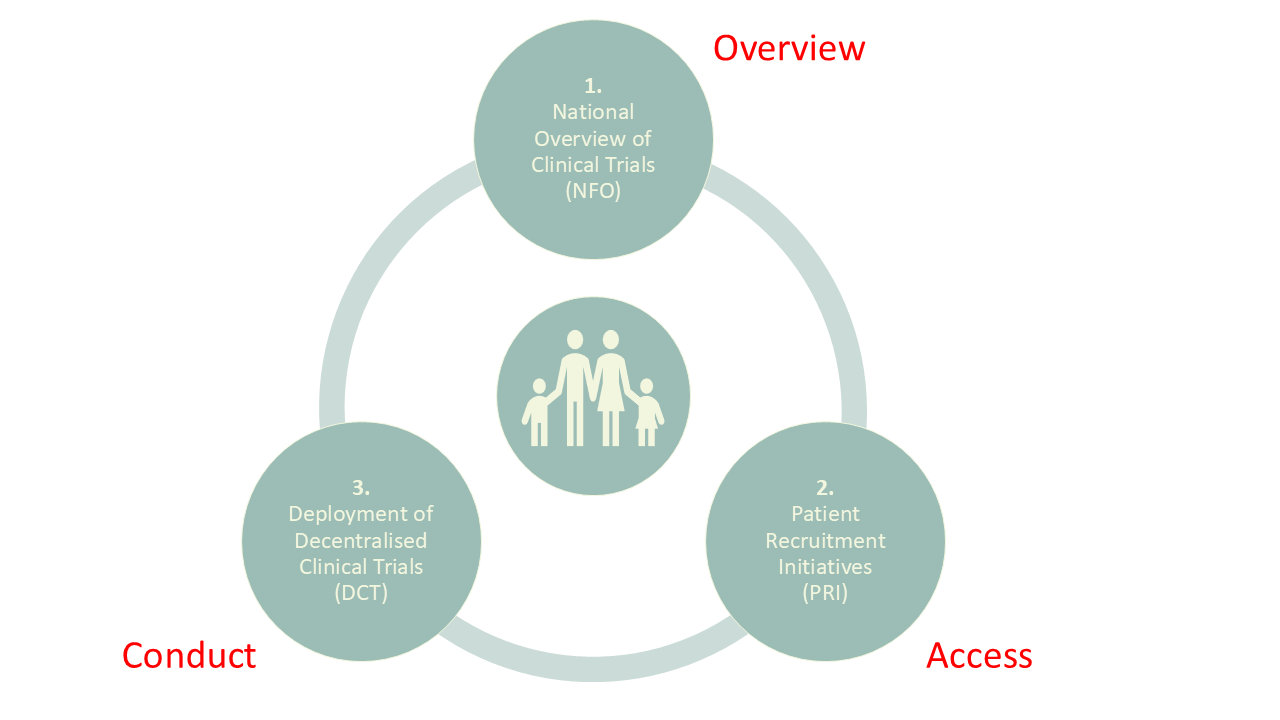
Health research should be for the benefit of everyone, thus establishing frameworks supporting a more patient-centered approach may increase equity in healthcare.

*Trial Nation, The five Danish regions, Aalborg University, IQVIA Solutions Denmark A/S, Novartis Healthcare A/S, Oticon A/S, World Courier – Specialty Logistics specialist, Merck A/S, Roche A/S and Novo Nordisk Denmark A/S.
PACT Key Focus Areas:
- Reducing Barriers: The project aims to lower the barriers for patients and healthcare professionals to participate in clinical trials, as well as for companies to conduct industry-initiated trials in Denmark.
- Enabling Remote Participation: PACT is focused on enabling patients to participate in clinical trials from their homes and/or local communities, enhancing convenience and accessibility.
- Fostering Patient-Centred Care: The project supports the development of a value-creating health care system focused on patient-centered care.
PACT Strategic Objectives:
- Digital Tools Implementation: Develop and implement digital tools that facilitate overview and access to clinical trials directly available for patients across Denmark.
- Public-Private Framework: Establish a sustainable public-private framework to support the patient-centred execution of decentralised clinical trials in Denmark.
- Increased Accessibility: Enhance the accessibility of clinical trials by increasing the number and proportion of patients participating in decentralised clinical trials.
Through these initiatives, the PACT project is committed to advancing clinical research in Denmark by centering the experience and needs of patients.

